Abstract
Introduction: In recent years, Venetoclax (VEN), a potent BCL2 inhibitor, in combination with hypomethylating agents (HMAs) has revolutionized treatment for Acute Myeloid Leukemia (AML) patients unfit for intensive induction chemotherapy. Heterogeneity in the efficacy of VEN/HMA therapy combined with better tolerability for patients compared to standard chemotherapy requires better biomarkers to select appropriate patients who would benefit from first-line VEN/HMA treatment rather than chemotherapy.
Aim: In this study we aimed to identify novel predictors of response to VEN/HMA by combining clinical, immune-phenotypic and genetic data with in vitro drug treatment, multiparameter BH3- and transcriptomic profiling as well as xenograft models to identify disease driving subpopulations.
Results: First, we confirm that a monocytic differentiation state either in monocytic-like cell lines or cultured primary patient samples is associated with resistance to ex vivo VEN/HMA treatment. In striking contrast, in our patient cohort of 54 first-line VEN/HMA patients, a monocytic differentiation state of the AML at diagnosis fails to translate into poor outcomes to VEN/HMA therapy conflicting with the data obtained using cultured cells. We hypothesized, that this is caused by the existence of a therapy-outcome determining AML subpopulation hidden to bulk level analysis. We find that expression of GPR56 in immature blasts distinguishes a conserved LSC-like population, present likewise in CD34+ and CD34- AMLs, that highly varies in size (0.5-90% GPR56+ within patient AML cells). Importantly, these GPR56+ LSC-like cells derived from both monocytic or primitive AML patient samples are the only population consistently engrafting in immunocompromised NSG mice (14/14 AML samples). Moreover, prospectively isolated LSC-like cells derived from primitive or monocytic AMLs are indistinguishable from one another based on RNA-sequencing, BCL2-family expression or apoptotic-dependency assessed by BH3-profiling. Vice versa, mature (CD11b+/CD64+) blasts from either monocytic or primitive samples are highly MCL1 but not BCL2 dependent and fail to consistently induce AML in NSG mice. By serial blood sampling of AML patients undergoing clinical VEN/HMA therapy, we validated that LSC-like cells are rapidly depleted, while mature blasts persist.
To study whether these findings translate into clinical outcomes, we treated diagnostic AML samples (n = 26) ex vivo with VEN/HMA and analyzed the effect on individual subpopulations. We found that in vitro killing of LSC-like cells, but not mature blasts, predicts actual clinical response of the same patients.
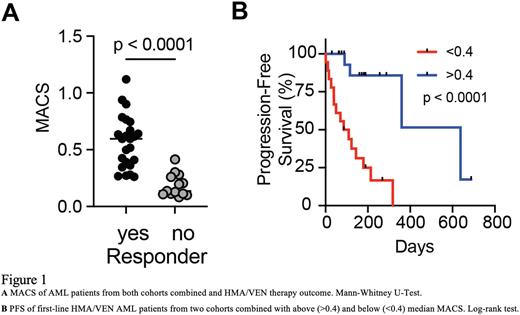
To further develop these findings into a flow cytometry-based biomarker, we generated the "Mediators of Apoptosis Combinatorial Score" (MACS) which takes advantage of the levels of BCL2, MCL1 and BCL-xL protein specifically in the LSC-like population. MACS predicted complete remission with a positive prediction score of 94% in two independent cohorts comprising 37 first line VEN/HMA patients (Figure 1A). MACS outperformed response prediction based on genetic, phenotypic and ex-vivo drug-screening . Prediction failed when unselected bulk AML cells instead of LSC-like cells were chosen as basis for MACS calculation, highlighting the importance of assessing the disease-driving population. Additionally, MACS predicted time on therapy and progression-free survival even within responders, enabling estimation when relapse is likely to occur (Figure 1B). Importantly, low MACS score was not associated with poor response to standard chemotherapy, pointing to standard chemotherapy as a superior treatment option for MACS-low, fit AML patients. The prospective validation of MACS is currently ongoing.
Finally, to provide therapeutic alternatives for MACS low patients likely to be VEN/HMA resistant, we modified MACS to predict therapeutic response to alternative BCL-2 family targeting drugs and were able to functionally validated the optimal predicted combinations of other BH3-mimetics including MCL1 and BCL-xL inhibitors for patient individualized treatment recommendations in 15 of 18 patients on LSC level.
In summary, MACS is an affordable, fast, translatable and versatile flow cytometry-based biomarker that identifies VEN/HMA-sensitive patients and provides personalized medicine in high-risk patients, potentially beyond AML.
Disclosures
Renders:Gilead: Consultancy, Honoraria. Unglaub:Jazz Pharmaceuticals: Other: travel costs. Shahswar:AbbVie: Honoraria; Jazz Pharmaceuticals: Honoraria. Heuser:Abbvie: Consultancy, Honoraria, Research Funding; Eurocept: Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Janssen: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Agios: Consultancy, Research Funding; BMS: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Glycostem: Consultancy, Research Funding; Kura Oncology: Consultancy; Pfizer: Consultancy, Research Funding; PinotBio: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Tolremo: Consultancy; Astellas: Research Funding; Bayer Pharma AG: Research Funding; BergenBio: Research Funding; Loxo Oncology: Research Funding. Schlenk:Daiichi Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; Pfizer: Honoraria, Research Funding; PharmaMar: Research Funding; AstraZeneca: Research Funding; Novartis: Honoraria; BergenBio: Honoraria. Hundemer:Sanofi: Research Funding; BMS: Research Funding; Novartis: Research Funding; Celgene: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees. Sauer:Pfizer: Honoraria; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria; Amgen: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Ridgeline Discoveries: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal